FDA Approves Talazoparib In Combination With Enzalutamide for mCRPC
The PARP inhibitor talazoparib has been approved in combination with enzalutamide for the treatment of adult patients with homologous recombination repair gene–mutated metastatic castration-resistant prostate cancer.
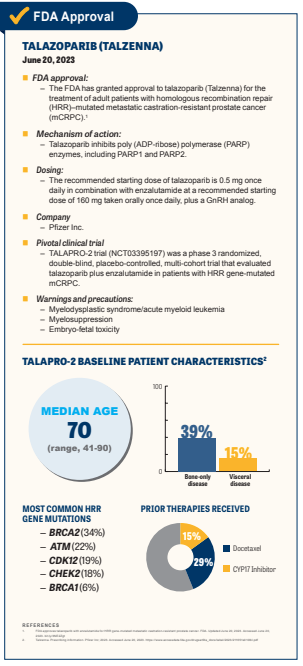
The FDA has approved talazoparib (Talzenna) in combination with enzalutamide (Xtandi) for the treatment of adult patients with homologous recombination repair (HRR)-mutated metastatic castration-resistant prostate cancer (mCRPC). The approval is based on data from the phase 3 TALAPRO-2 trial (NCT03395197).1,2
The median radiographic progression-free survival (rPFS) with talazoparib/enzalutamide was not estimable (NE) (95% CI, 21.9-NE) vs 13.8 months (95% CI, 11.0-16.7) with placebo/enzalutamide (HR, 0.45; 95% CI, 0.33-0.61; P < .0001).1,2
Investigators of TALAPRO-2 assessed for one of the following HRR gene mutations before randomly assigning patients to an investigative or control arm: ATM, ATR, BRCA1/2, DK12, CHECK2, FANCA, MLH1, MRE11A, NBN, PALB2, or RAD51C. These were identified using tissue-based or circulating tumor DNA assays.2 Prior to enrollment it was also required that patients had undergone prior orchiectomy or received gonadotropin-releasing hormone (GnRH) analogs if the procedure was not completed. Additionally, patients must have experienced disease progression on androgen deprivation therapy.1,2
The radiographic progression-free survival (rPFS) with talazoparib/enzalutamide was not estimable (NE) (95% CI, 21.9-NE) vs 13.8 months (95% CI, 11.0-16.7) with placebo/enzalutamide (HR, 0.45; 95% CI, 0.33-0.61; P < .0001).
Findings from TALAPRO-2 also showed that when stratified by BRCA status, patients with BRCA-mutant mCRPC had a median rPFS of NE (95% CI, NE-NE) vs 11.0 months (95% CI, 8.3-11.1) with talazoparib vs placebo, respectively (HR, 0.20; 95% CI, 0.11-0.36). Those with non–BRCA-mutated mCRPC had a median rPFS of 24.7 months (95% CI, 16.4-NE) vs 16.7 months (95% CI, 13.8-27.7) with the investigative and control regimens, respectively (HR, 0.72; 95% CI, 0.49-1.07).2
The recommended starting dose of talazoparib is 0.5 mg once daily in combination with enzalutamide at a recommended starting dose of 160 mg taken orally once daily and with a GnRH analog unless bilateral orchiectomy.
Dose reductions are permitted to manage adverse effects (AEs) and include the following guidelines: first dose reduction 0.35 mg once daily; second dose reduction 0.25 mg once daily; and third dose reduction 0.1 mg once daily.
Commonly reported AEs included fatigue, nausea, fractures, dizziness, and dysgeusia. The label comes with warning and precautions for myelodysplastic syndrome/acute myeloid leukemia, myelosuppression, and embryo-fetal toxicity.1
References
- FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. FDA. June 20, 2023. Accessed June 20, 2023. bit.ly/3NE2Zgt
- Talzenna. Prescribing information. Pfizer Inc, 2023. Accessed June 20, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/211651s010lbl.pdf



