Preparing Patients for CAR T-Cell Therapy With Confidence
As the therapy continues to evolve, best nursing practices become more nuanced.
Over the past 6 years, a form of immunotherapy known as chimeric antigen receptor (CAR) T-cell therapy has emerged as a promising approach in certain blood cancers that don’t respond to other treatments.
Dara Feleciano, MSN, RN, CNS, OCN, BMTCN

Dara Feleciano, MSN, RN, CNS, OCN, BMTCN, a clinical research nurse in the Alpha Stem Cell Clinic at the UC Davis Comprehensive Cancer Center in Sacramento, explained that the therapy uses a patient’s own immune system to fight cancer.
“We’re essentially giving patients a ‘living drug’ that supercharges their T cells, turning them into cellular therapy that recognizes and targets cancer cells,” she told Oncology Nursing News®, and thereby offering “many patients with cancer sustained remissions.”
The process begins with the extraction T cells, a type of white blood cell, from patients during a blood filtration procedure that takes 2 to 3 hours to complete. The cells, Feleciano said, are then frozen and sent to a special laboratory where they are reengineered through the addition of CAR protein to them over the course of several weeks. After the new, reprogrammed T cells have grown and multiplied, they are given back to patients via intravenous (IV) infusion and work to target and destroy their cancer cells.
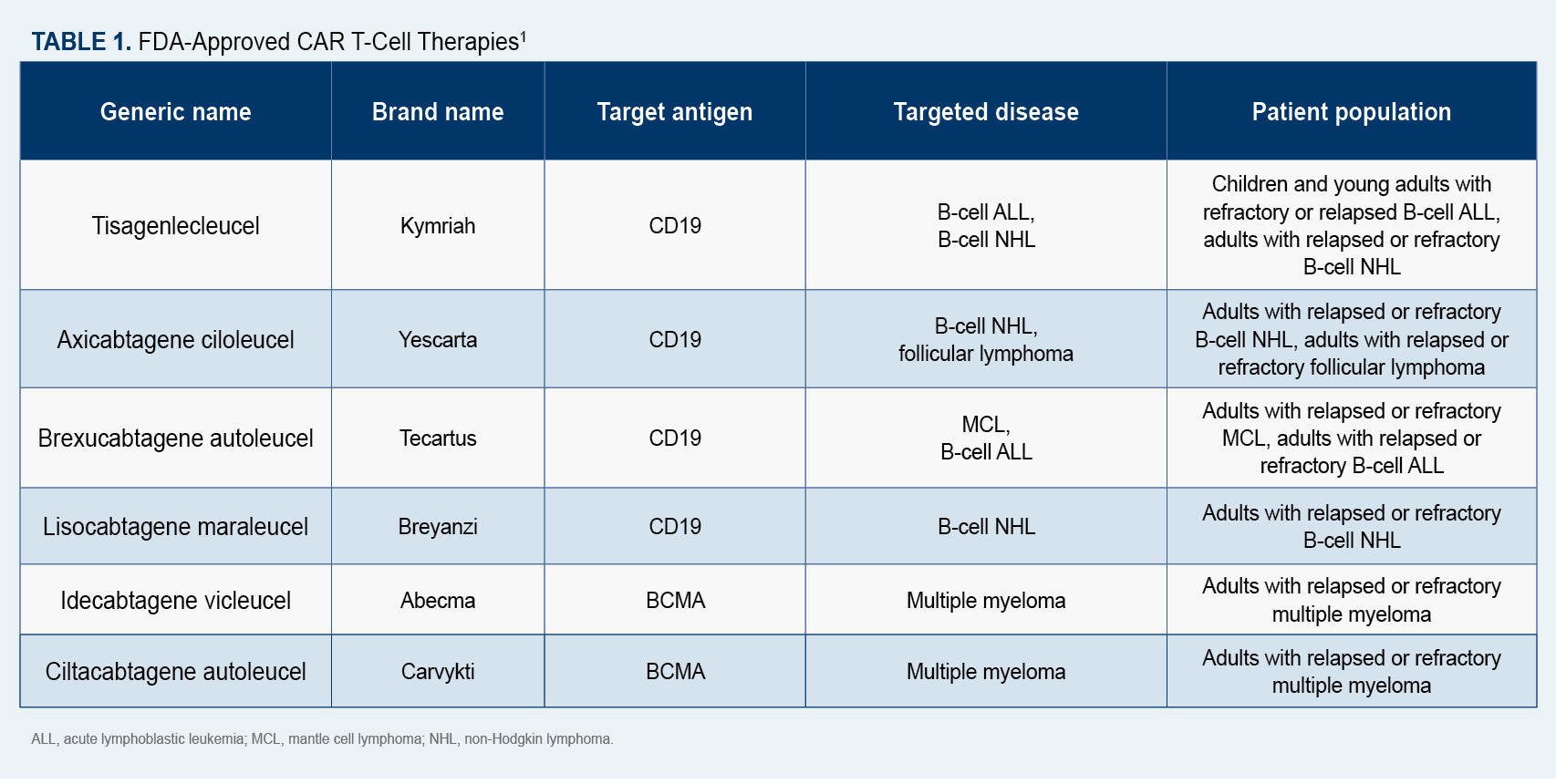
Since 2017, 6 CAR T-cell therapies (Table 11) have been approved by the FDA for the treatment of blood cancers, including multiple myeloma, certain lymphomas, and some forms of leukemia.1
Identifying Good Candidates
Until last year, CAR T-cell therapy was considered a last resort for patients who had previously undergone chemotherapy and a stem cell transplant. That changed in April 2022 when the FDA approved axicabtagene ciloleucel (Yescarta) for use in patients with large B-cell lymphoma after only 1 treatment failure.2 There are now several clinical trials underway to determine whether other CAR T-cell agents can be used safely and effectively and may be offered to patients earlier in their cancer care.
Feleciano noted that a lot of work goes into preparing patients for CAR T-cell therapy, beginning with a wide array of tests that are conducted approximately 30 days before they are scheduled to receive treatment. “
Patients have their overall health, function, and fitness level evaluated through… bloodwork, an EKG, echocardiogram, a bone marrow aspiration, and a PET scan to determine if they’re a good candidate for CAR T-cell therapy,” she said, adding that patients also meet with the hospital’s financial services department to ensure their insurance covers a portion of the therapy.
“Since CAR T-cell therapy is only offered at a limited number of cancer centers, many patients incur travel and lodging costs,” Feleciano pointed out. “There are financial assistance programs available that can sometimes help with the costs of lodging for them and their caregivers, since we require them to stay within 60 minutes of UC Davis Health for a month after their procedure.”
Because it takes time for the CAR T cells to be reprogrammed in the laboratory, in some cases patients are offered bridging therapy—which can include high-dose steroids, chemotherapy, or radiation— to control disease before CAR T-cell therapy begins.
Preparing the Patient
Three days before receiving CAR T-cell therapy, patients are administered lymphodepleting chemotherapy to reduce their level of white blood cells, thus increasing the effectiveness of the new CAR T cells, Feleciano said. “The modified CAR T-cells are thawed at a patient’s bedside and administered in a single IV infusion that typically takes 30 minutes or less.” Patients may be hospitalized for a week after infusion so that their response to treatment can be monitored and potential side effects treated.
“CAR T-cell therapy has proven to be a good alternative for patients with aggressive blood cancers, with many patients achieving complete remission,” she stated.
Recognizing Adverse Effects
Although the therapy has proven effective in many patients who have failed to respond to other treatments, it comes with potentially serious adverse effects (AEs). Nurses administering it are required to undergo additional training in risk evaluation and mitigation and learn to recognize early signs and symptoms of AEs.
Maritza Alencar, DNP, MBA, APRN-BC, BMTCN, FAANP

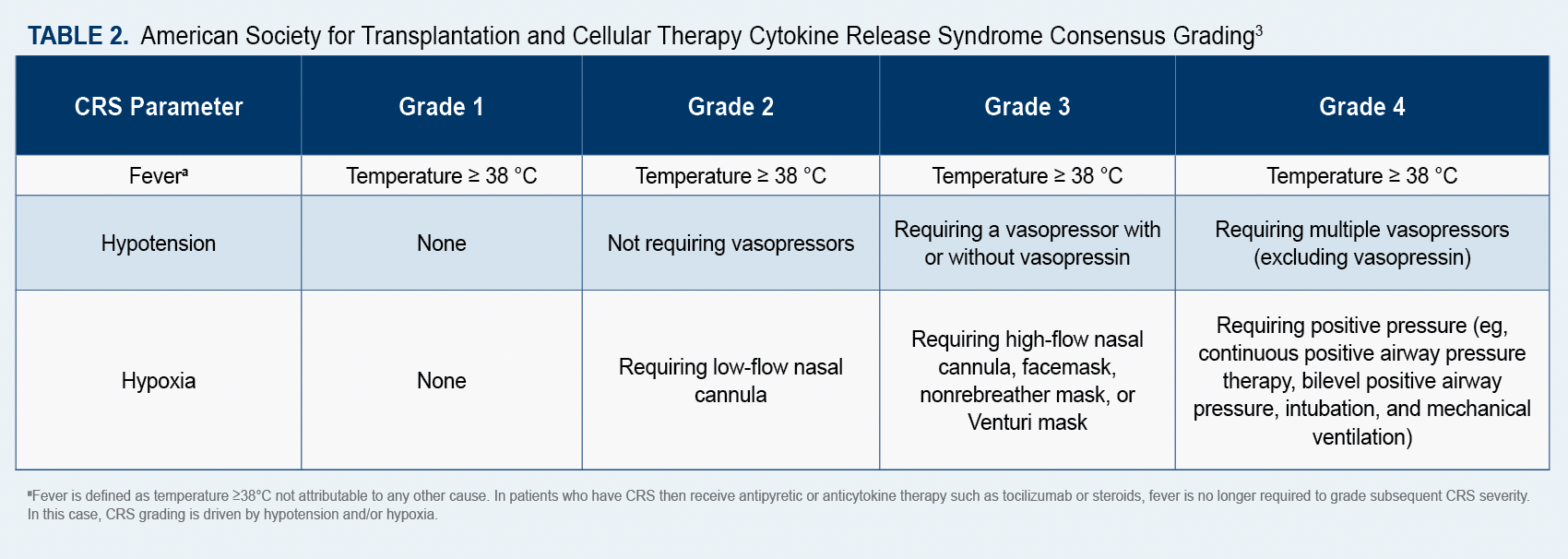
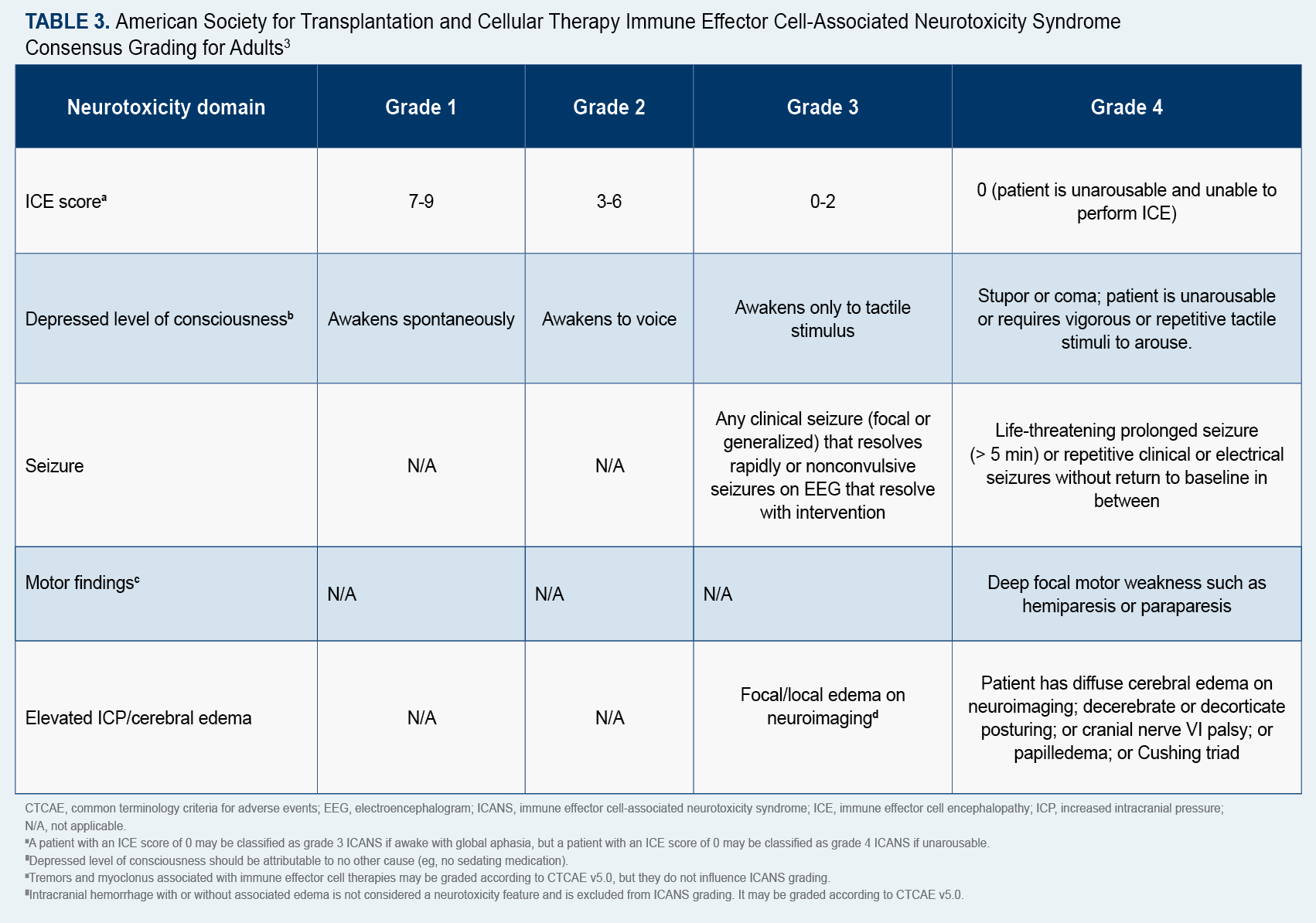
Maritza Alencar, DNP, MBA, APRN-BC, BMTCN, FAANP, executive director of clinical operations at University of Miami Sylvester Comprehensive Cancer Center in Florida, and current co-chair for the American Society for Transplantation and Cellular Therapy (ASTCT) Advanced Practice Provider Special Interest Group (SIG), told Oncology Nursing News® that because “patients can present with symptoms of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS), it is important for nurses to understand potential AEs and to monitor patients for subtle changes…after they receive CAR T-cell therapy.”
ICANS, which typically occurs within a week of infusion, can present with a variety of neurological symptoms, she said, including headache, tremor, speech impairment, delirium, and confusion. In contrast, CRS can cause such flu-like symptoms as high fever, fatigue, headache, dizziness, and a fast heart rate. She added that patients are given corticosteroids to combat AEs of ICANS and the immunosuppressive drug, tocilizumab, for AEs of CRS.
“Nurses need to know how to assess, identify, and understand what potential management would entail for neurotoxicities,” Alencar said, and “the earlier these symptoms are identified, the sooner they can be treated, leading to a better outcome.”
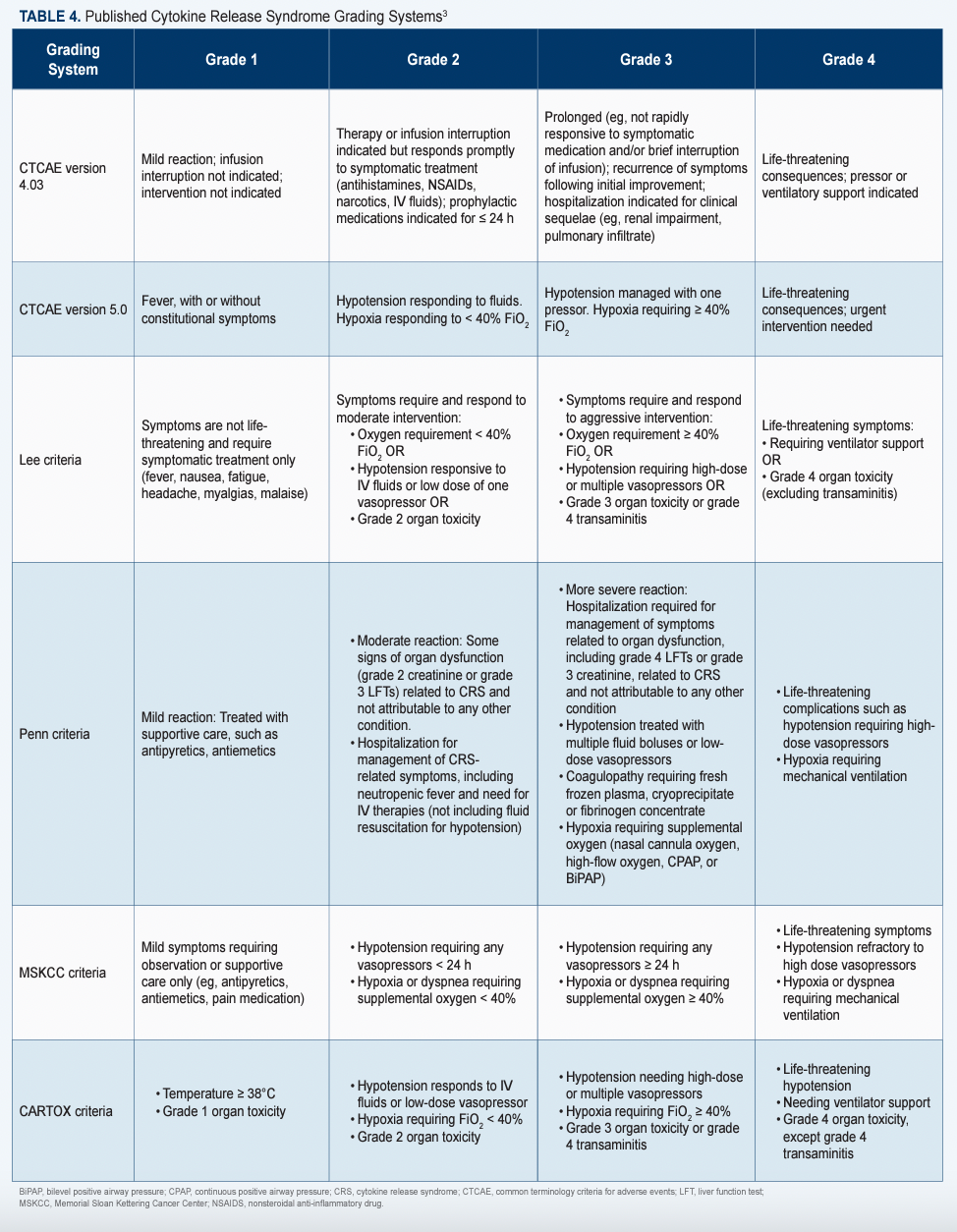
The ASTCT has established consensus grading criteria for CRS and ICANS, which are available online (Table 23; Table 33),and recommends that a 10-point screening tool called ICE be used in conjunction with frequent monitoring to detect early signs of ICANS.4
To help nurses better identify and manage CAR T-cell AEs, Sherry Adkins, MSN, an advanced practice provider supervisor in the Lymphoma/Myeloma Department at The University of Texas MD Anderson Cancer Center, in Houston, Texas, developed a mobile app, CARTOX, which may be downloaded free of charge from the Apple Store and Google Play. Feleciano noted that 85% of patients experience CRS, which can range in severity from low-grade symptoms that are manageable with supportive care to higher-grade symptoms that can prove life-threatening without treatment.
“CAR T-cell therapy ignites a patient’s immune system, but it can also sometimes be overwhelming to their body, leaving them vulnerable to AEs, such as CRS and infection,” she explained.
Since CRS symptoms can present up to 8 weeks after infusion, Alencar said it’s also critical for nurses to teach patients and caregivers to recognize the subtle changes that indicate someone may be experiencing side effects (Table 43). She also expressed the importance for patients and caregivers to stay near the treating center and avoid driving or using any heavy machinery that could be dangerous for the patient experiencing cognitive impairment.
“Even if patients don’t experience AEs in the hospital, there’s a chance they could experience them after they’re discharged,” she added. “For this reason, it’s important to provide education on potential side effects and ensure patients attend their follow-up appointments once or twice a week…. They should also have access to a call service they can contact if they have concerns.”
In addition, researchers are hoping to find other ways to identify toxic side effects at the earliest stages. A recent study by the American Association for Cancer Research Journal, found that monitoring a patient’s phosphorus levels may be an effective way of anticipating the development of ICANS. Investigators discovered that decreases in serum phosphorus levels correlate with the incidence and severity of ICANS. They are also continuing to look for ways to identify patients who may be at greater risk of AEs.
A new study conducted at Washington University School of Medicine in St. Louis, and published September 1 in JAMA Oncology, showed that a simple blood test administered before CAR T-cell therapy may indicate which patients are predisposed to developing neurotoxic side effects. The study found that levels of the neurofilament light chain protein are higher in patients who go on to develop neurotoxic side effects after CAR T-cell therapy. 5
The Future of the Therapy
Although CAR T-cell therapy is a game changer for many oncology patients and has resulted in a significant number of longterm remissions, Alencar noted that it’s only being used in certain blood cancers and offered at approximately 150 medical centers around the country.6 Some centers, including The University of Chicago Medicine Comprehensive Cancer Center, in Illinois, have also begun offering CAR T-cell therapy on an outpatient basis.6
Data released last year by Novartis, the manufacturer of tisagenlecleucel (Kymriah), the first approved CAR T-cell therapy for patients with relapsed or refractory B-cell acute lymphoblastic lymphoma, found that 55% of patients who had undergone CAR T-cell therapy were alive 5 years after treatment. In addition, 44% of patients who experienced remission within 3 months of receiving tisagenlecleucel were still in remission at the 5-year mark.7 A similar study published last year in The New England Journal of Medicine, found that idecabtagene vicleucel (Abecma), a CAR T-cell therapy used to treat multiple myeloma, led to a response in almost 75% of patients who had relapsed after several previous treatments.8
“Clinical trials are now looking at how CAR T-cell therapy might treat solid tumors such as lung, breast, pancreatic, and ovarian cancers,” Alencar said.
Researchers are also exploring ways to improve long-term outcomes for patients who receive CAR T-cell therapy and possibly establishing it as a first-line treatment in the future.
Furthermore, many cancer centers, including UC Davis Health, in California, and Penn Medicine, in Philadelphia, Pennsylvania, are working to accelerate the CAR T-cell manufacturing process. Feleciano said UC Davis Health is producing CAR T cells on-site, thus reducing storage and transportation costs as well as the time it takes for patients to receive treatment and how much they pay for it.
A recent study showed that a team at the Perelman School of Medicine at the University of Pennsylvania, in Philadelphia, shortened the process, which typically takes 9 to 14 days, to just 24 hours.9 Many clinicians are optimistic about the potential impact of reducing production time.
“Timing is everything when it comes to CAR T-cell therapy,” Alencar says. “If we can shorten the timing to referral and determine if a patient may be eligible for CART-cell therapy, then it allows more time for adequate CAR T-cell arrangements and optimal timing for treatment leading to improved patient outcomes and a decrease in overall cancer burden.”
References
- CAR T-cells: engineering patients’ immune cells to treat their cancers. National Cancer Institute. Updated March 10, 2022. Accessed January 6, 2023. https://bit.ly/3XeumQk
- Golden, D. Yescarta impresses as first-line therapy option for large B-cell lymphoma. Alliance for Cancer Gene Therapy. August 2, 2022. Accessed January 6, 2023. https://bit.ly/3impMAW
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. doi:10.1016/j.bbmt.2018.12.758
- Ellard R, Kenyon M, Hutt D, et al. The EBMT immune effector cell nursing guidelines on CAR-T therapy: a framework for patient care and managing common toxicities. Clin Hematol Int. 2022;4(3):75-88. doi:10.1007/s44228-022-00004-8
- Butt OH, Zhou AY, Caimi PF, et al. Assessment of pretreatment and posttreatment evolution of neurofilament light chain levels in patients who develop immune effector cell-associated neurotoxicity syndrome. JAMA Oncol. 2022;8(11):1652-1657. doi:10.1001/jamaoncol.2022.3738
- Medical centers that offer CAR T-cell therapy. BMT Infonet. Accessed January 6, 2023. https://bit.ly/3WZhDRJ
- Novartis five-year Kymriah data show durable remission and long-term survival maintained in children and adults with advanced B-cell ALL. News release. Novartis. June 12, 2022. Accessed January 6, 2023. https://bit.ly/3GNjmTF
- Munshi NC, Anderson LD Jr, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory myeloma. N Engl J Med. 2021;384(8):705-716. doi:10.1056/NEJMoa2024850
- Ghassemi S, Durgin JS, Nunez-Cruz S, et al. Rapid manufacturing of non-activated potent CAR T cells. Nat Biomed Eng. 2022;6(2):118-128. doi:10.1038/s41551-021-00842-6

Shared Model of Care Post-HCT Offers Safe Follow-Up, Reduces Patient Burden
Published: March 19th 2025 | Updated: March 19th 2025Alternating post-HCT care between specialized facilities and local cancer centers produced noninferior non-relapse mortality and similar quality of life to usual care.



