Caring for a Patient Undergoing CAR T-Cell Therapy for Relapsed/Refractory Multiple Myeloma
The introduction of CAR T-cell therapy for patients with relapsed/refractory multiple myeloma is considered one of the most significant advancements in the past decade.
Nicole McEntee, BSN, RN, OCN, BMTCN

The introduction of CAR T-cell therapy for patients with relapsed/refractory multiple myeloma is considered one of the most significant advancements in the past decade.1 In March 2021, the FDA approved the first CAR T-cell therapy for relapsed or refractory multiple myeloma after 4 or more lines of therapy.2 Idecabtagene vicleucel (Abecma) targets the b-cell maturation antigen (BCMA), which is found to be expressed on the surface of malignant myeloma cells, and was approved that year to treat patients with relapsed or refractory multiple myeloma after 4 or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody.2,3
CAR T-cell therapy uses a patient’s own immune system to fight their cancer. The patient’s T cells are collected, sent to a laboratory or manufacturing facility to be genetically engineered and expanded, and returned to the treatment center where they are infused back into the patient following a brief course of lymphodepleting chemotherapy.4
Identifying patients who could benefit from CAR T-cell therapy early and initiating the referral process to a certified treatment center could optimize patient outcomes. The process of evaluation through infusion of the CAR T cells can take weeks to months as manufacturing time is approximately 28 days.5
Patient Case
Frontline Treatment Approaches
Ms C is a 63-year-old woman who presented with symptomatic bone disease and anemia in August 2010. She was diagnosed with multiple myeloma with high-risk cytogenetic and chromosomal abnormalities. Ms C was initially treated with bortezomib (Velcade) plus dexamethasone (Decadron) for 1 cycle, followed by VRd (bortezomib, lenalidomide [Revlimid], and dexamethasone) for 3 cycles. In February 2011, she had autologous peripheral stem cell infusion. She was started on maintenance Revlimid until January 2019 when her M protein started to rise and ixazomib (Ninlaro) and dexamethasone were added.
APET-CT in May 2020 showed disease progression in the thoracic and lumbar spine. Her therapy was consequently changed to daratumumab (Darzalex), dexamethasone, and pomalidomide. After experiencing worsening back pain with accompanying numbness and tingling, she went to the emergency department for evaluation. There, a CT scan of her spine showed progressive pathologic fractures and lytic metastases. She therefore received radiation to her spine. In January 2021, after experiencing shoulder pain, an x-ray showed pathologic fractures and a metastatic lesion to the right humerus. She continued radiation to her spine, and right arm and shoulder. She was treated with carfilzomib and dexamethasone from February to May, when her treatment was changed to D-PACE (dexamethasone, cisplatin, doxorubicin, cyclophosphamide, and etoposide).
Transition to CAR T
In early July, our patient achieved a very good partial response, at which point she had a second autologous stem cell infusion. A PET-CT in November 2021 demonstrated that she had a significant response to therapy. However, because of the aggressiveness of her disease, we knew she would not remain in remission without further therapy. So, she underwent lymphocyte collection in preparation for CAR T-cell therapy.
Ms C received lymphodepleting chemotherapy in the ambulatory infusion unit and was admitted for the CAR T-cell infusion in December 2021. Our standard of care includes prophylactic anti-viral, anti-fungal, antibiotic, and anti-seizure medications. Additionally, we assess patients for adverse events including cytokine release syndrome (CRS) and immune-effector cell-associated neurotoxicity syndrome.
Successful Management
Our patient tolerated the infusion well. However, the following morning she developed a fever of 100.8 °F. Since her blood pressure and oxygen saturation were within normal limits, we determined that she had grade 1 CRS. An infectious work-up was done, at which point her levofloxacin (Levaquin) was changed to cefepime (Maxipime).
Later that day, she had a second fever of 100.4 °F. We managed that fever with tocilizumab (Actemra). She did not have any additional fevers or symptoms of CRS.
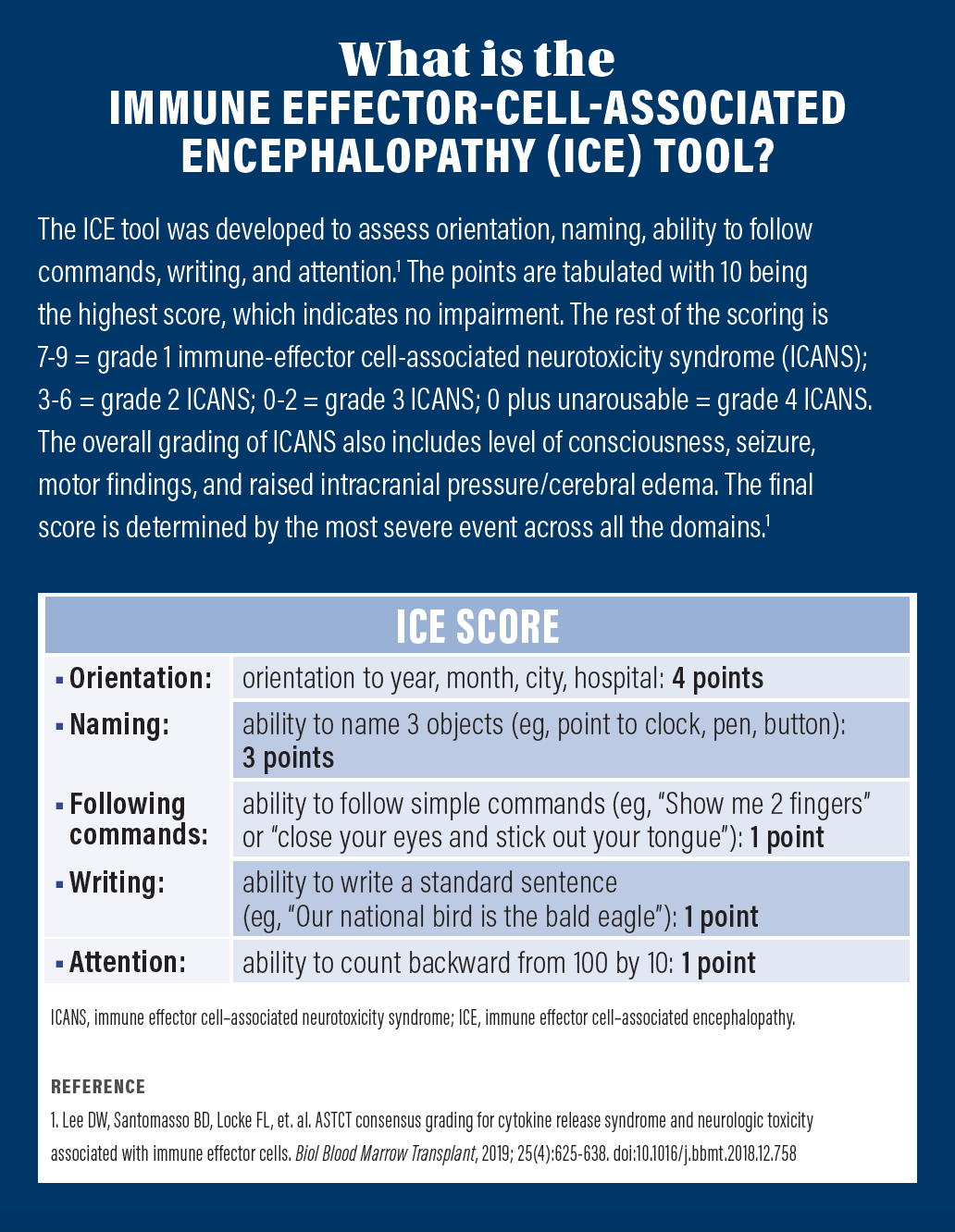
Throughout the duration of her admission, she did not have any neurotoxicity symptoms and her immune-effector cell-associated encephalopathy score was 10. On day 9, she was discharged and followed in the ambulatory clinic. In February 2022, her PET scan showed no evidence of active or progressive multiple myeloma. She continues to be regularly followed in the clinic.
Implications for Practice
Currently, all CAR T-cell products have black box warnings for CRS and neurologic toxicities. These adverse events have the potential to become severe, so it is important that institutions establish clear usage guidelines. For years, the goal of therapy for patients with multiple myeloma was disease stabilization. The introduction of CAR T-cell therapy into the treatment paradigm has given these patients hope for a complete response.
References
- Van Oekelen O, Nath K, Mouhieddine TH, et. al. Interventions and outcomes of patients with multiple myeloma receiving salvage therapy after BCMA-directed CAR T therapy. Blood. 2023;141(7):756-765. doi:10.1182/blood.2022017848
- FDA approves idecabtagene vicleucel for multiple myeloma. News release. FDA. March 29, 2021. Accessed October 9, 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-idecabtagene-vicleucel-multiple-myeloma
- Guo R, Lu W, Zhang Y, Cao X, Jin X, Zhao M. Targeting BCMA to treat multiple myeloma: updates from the 2021 ASH annual meeting. Front Immun. 2022:13(839097). doi:10.3389/fimmu.2022.839097
- Chimeric antigen receptor (CAR) T-cell therapy. The Leukemia & Lymphoma Society. Accessed June 7, 2023. https://www.lls.org/treatment/types-treatment/immunotherapy/chimeric-antigen-receptor-car-t-cell-therapy
- Rendo MJ, Joseph JJ, Phan LM, DeStefano CB. CAR T-cell therapy for patients with multiple myeloma: current evidence and challenges. Blood Lymphat Cancer. 2022;12:119-136. doi:10.2147/BLCTT.S327016
- Lee DW, Santomasso BD, Locke FL, et. al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant, 2019; 25(4):625-638. doi:10.1016/j.bbmt.2018.12.758




