Rapid-Response System Enhances Palliative Care, Lowers Costs
A collaborative patient reported outcome (PRO)-based care approach resulted in better outpatient symptom management and a decrease in end-of-life hospitalizations and costs for late-stage cancer patients.
Paul W. Read, MD, PhD
Professor,
Radiation Oncology,
University of Virginia
A collaborative patient reported outcome (PRO)-based care approach resulted in better outpatient symptom management and a decrease in end-of-life hospitalizations and costs for late-stage cancer patients. The results of a 3-year pilot clinical research program were recently presented at the 57th Annual Meeting of the American Society for Radiation Oncology (ASTRO) by a University of Virginia (UVA) palliative care and radiation oncology team.
If we listen to our patients carefully, talk to them about their changing medical and emotional needs, and develop rapid and coordinated treatment plans, we can improve their quality of life and reduce their need for hospitalization for symptom management at the end of life. Integrating patient surveys to collect patient reported outcomes directly into electronic medical records and incorporating them into routine clinical care can be done in most hospital systems.
Leslie Blackhall, MD, associate professor of Geriatrics and Palliative Care at UVA, piloted the new palliative care program at the Emily Couric Clinical Cancer Center in 2012 for patients with advanced cancer by integrating an NIH PROMIS-based PRO database named MyCourse and tested the patient’s ability to complete this questionnaire. MyCourse tracks emotional and physical well-being as reported by the patient, which is displayed longitudinally in the electronic medical record and is accessible to all healthcare providers showing the course of symptoms during the late stages of illness.
If certain symptoms, such as pain, escalate to a specified level, the system triggers an email alert that is sent to the nurse coordinator of a comprehensive assessment and rapid evaluation and treatment (CARE Track) palliative care team for rapid intervention. The CARE Track team meets weekly during a supportive care tumor board to develop rapid and coordinated care plans for highly symptomatic patients in an effort to respond more quickly to their needs.
Overall, 646 patients were enrolled into the CARE Track program over 30 months. Researchers compared end-of-life data from 368 deceased CARE Track patients with 198 patients in a cohort of matched deceased institutional controls (Table).
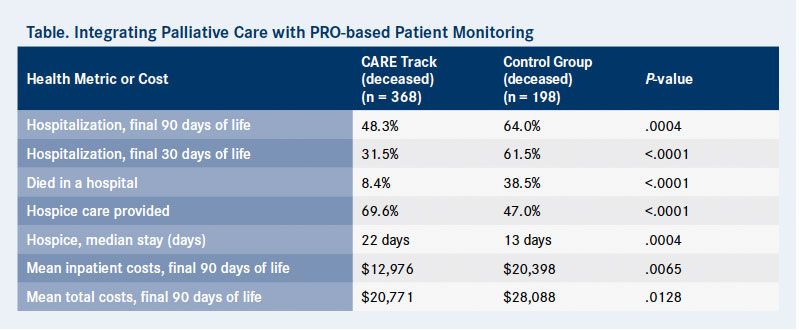
The 368 deceased CARE Track patients completed 967 patient-reported surveys. This cohort had significantly fewer end-of-life hospitalizations than controls; 48.3% were hospitalized within the final 3 months of life compared with 64% of the control arm (P = .0004). Further, more CARE Track patients received hospice care (69.6% vs 47%) and spent more days in hospice (median stay, 22 days vs 13 days; P = .0004) than patients in the control group. This resulted in fewer hospital deaths for the CARE Track patients compared with controls (8.4% vs 38.5%; P < .0001).
Results of a cost analysis showed these reductions in hospitalizations and hospital deaths decreased the mean total cost of care per patient by $7317 in the final 90 days of life (P = .0128). Mean inpatient costs in the final 90 days of life also were significantly lower ($12,976 vs $20,398; P = .0065).
To respond more quickly to pain, the UVA department of Radiation Oncology developed STAT RAD—a more rapid workflow for palliative radiation therapy for patients with bone metastases with the goal of turning a common two-to-three week treatment course into a 1-day treatment procedure with a highly focused radiation treatment (RT) to reduce treatment-related toxicity.
The STAT RAD pilot clinical trial enrolled 28 patients. The patients each had between one and three painful bone metastases (37 target lesions) and received RT of 5 Gy to 10 Gy per fraction, for between 2 and 5 fractions. An average of 21.6 Gy in 3.1 fractions was administered.
The pain response of patients in the STAT RAD program was assessed using the International Bone Metastasis Consensus Working Party. The patients’ quality of life (QoL) was assessed using the Functional Assessment of Cancer Therapy— Bone Pain Scale. Patients reported 80% to 90% partial or complete pain relief by 3 months and QoL was significantly improved for patients for a timeframe ranging from 1 week post STAT RT to 26 weeks post STAT RAD. A second clinical trial is still accruing patients and is exploring single- fraction STAT RAD with dose escalation from 8 Gy to 15 Gy so that the entire course of simulation, planning, quality assurance, and treatment can be completed in a single 4-hour patient-centric procedure.
Collaborative palliative care and radiation oncology teams can re-engineer patient centric workflows to improve health, healthcare, and reduce end-of-life costs. Gains are achieved through earlier palliative care integration with PRO-based patient monitoring and alerting and more efficient multidisciplinary care including rapid high-dose conformal radiation for patients with painful bone metastases.
The concept of tumor boards for multispecialty care planning of curative cancer patients is practiced throughout the country, and extending this concept to palliative care management is easy and straightforward. Single-fraction radiation therapy for palliation of the bone metastases for advanced cancer patients with short life expectancies is an accepted national care guideline and has been studied for decades in clinical trials. Therefore, these programs can all be adopted into clinical practice at most health systems with minimal cost, training, or education.
The project described was supported by Grant Number 1C1CMS331031 from the Department of Health and Human Services, Centers for Medicare & Medicaid Services. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Health and Human Services or any of its agencies. The research presented here was conducted by the awardee. Findings might or might not be consistent with or confirmed by the findings of the independent evaluation contractor.



