Palbociclib Combo Effective in Frontline Treatment of ER+, HER2- Postmenopausal Metastatic Breast Cancer
Palbociclib (Ibrance), a CDK 4/6 inhibitor, demonstrated significant efficacy in combination with the aromatase inhibitor letrozole (Femara) in the frontline setting of estrogen receptor (ER)-positive, HER2-negative, postmenopausal metastatic breast cancer in phase III PALOMA-2 study.
Palbociclib (Ibrance), a CDK 4/6 inhibitor, demonstrated significant efficacy in combination with the aromatase inhibitor letrozole (Femara) in the frontline setting of estrogen receptor (ER)-positive, HER2-negative, postmenopausal metastatic breast cancer. These findings were a result of a deeper analysis of the phase III PALOMA-2 study presented at the 2018 Miami Breast Cancer Conference®.
Across the full study, the combination of palbociclib plus letrozole significantly improved median progression-free survival (PFS) versus placebo plus letrozole in the first-line setting for metastatic disease (24.8 versus 14.5 months; HR, 0.58; 95% CI, 0.46-0.72; P <.001). For the added analysis, investigators concluded that palbociclib plus letrozole also “consistently and significantly” prolonged median PFS compared with placebo plus letrozole in all subgroups, regardless of prior adjuvant/neoadjuvant endocrine therapy or chemotherapy.
For patients with prior endocrine therapy, median PFS for the palbociclib-plus-letrozole group (n = 249) was 22.2 months versus 11.3 months for the placebo-plus-letrozole cohort (n = 126) (hazard ratio [HR], 0.53; 95% CI, 0.40-0.70). The objective response rate (ORR) was 33.7% versus 27.0%, respectively. In the no-prior endocrine therapy group, the median PFS was 25.7 months for the palbociclib group (n = 195) versus 19.6 months for the control group (n = 96) (HR, 0.63; 95% CI, 0.44-0.90). The respective ORR rates were 52.8% and 44.8%.
Among those treated with prior chemotherapy, the median PFS was 22.4 months (n = 213) versus 13.7 months for the non-palbociclib group (n = 109) (HR, 0.53; 95% CI, 0.40-0.72). The ORRs were 36.2% versus 30.3%, respectively. In the no-prior chemotherapy subgroup, median PFS was 25.7 months (n = 231) versus 17.0 months (n = 113) (HR, 0.61; 95% CI, 0.44-0.84). ORR was 47.6% versus 38.9% for the palbociclib-plus letrozole versus control.
“In the subgroup analysis, we evaluated benefit based on prior endocrine and prior chemotherapy, both of which would have been given in the neoadjuvant or adjuvant setting," lead author of the subgroup analysis Richard Finn, MD, from the David Geffen School of Medicine at UCLA, told OncLive®, a sister publication to Oncology Nursing News®. "The take-home message is that regardless of these prior therapies, there is still a significant improvement tin PFS with the addition of palbociclib to letrozole.”
"There are now 3 CDK 4/6 inhibitors approved in this indication, but only PALOMA-2 included patients who relapsed on prior endocrine therapy in less than 12 months, as long as it was not a non-steoridal AI," he added.
The study enrolled 666 postmenopausal women from February 2013 to July 2014 and randomly assigned them 2:1 to the palbociclib plus letrozole arm (n = 444) or the placebo plus letrozole arm (n = 222). The median age was between 58 and 65 years, and most patients were white (75%-81%) and non-Hispanic or Latino.
The most common prior endocrine therapies received were tamoxifen (22%-84%), anastrozole (6%-23%), letrozole (4%-15%), and exemestane (2%-12%). The median average daily palbociclib dose was 125 mg across all subgroups; the median number of cycles was 18 versus 21 for those receiving prior endocrine therapy versus those who did not, and 19 versus 21 for prior chemotherapy versus without, respectively.
The observed clinical benefit rate was 81.5% (palbociclib) versus 66.7% (placebo) for the prior endocrine therapy patients; 89.2% versus 75.0% for no prior endocrine therapy; 81.7% versus 70.6% for prior chemotherapy; and 87.9% versus 69.9% for no prior chemotherapy.
Median duration of response across subgroups receiving palbociclib plus letrozole was strongest among those with no prior therapy: 28.0 months for those with no prior endocrine therapy versus 22.5 months for pretreated patients, and 28.0 months for those with no prior chemotherapy versus 20.1 months for the pretreated group.
CAPTION
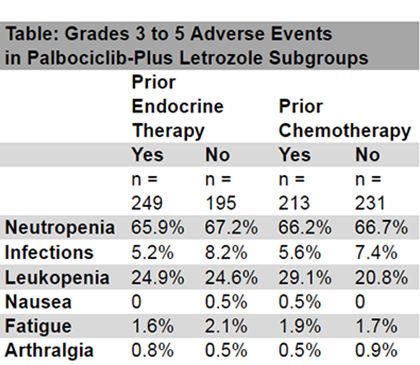
In the analysis, investigators found that tolerability was consistent across the subgroups and in accord with the known safety profile of palbociclib when combined with endocrine therapy. The most common treatment related adverse events were neutropenia, infections, leukopenia, nausea, fatigue, and arthralgia (Table).
Among all palbociclib plus letrozole groups, treatment emergent AEs of any grade leading to permanent discontinuation occurred in <10% of patients, regardless of prior therapy or type of therapy if pretreated. Permanent discontinuations due to grades 3 to 5 AEs ranged from 0.8% to 3.5%, and similar frequencies occurred in all subgroups. No new or unexpected safety issues were observed in this analysis.
Palbociclib has been explored across several settings for patients with ER-positive advanced breast cancer. The CDK4/6 inhibitor is approved in combination with an aromatase inhibitor as a frontline therapy and in combination with fulvestrant as a second-line therapy, both for postmenopausal women with HER2-negative, ER-positive metastatic breast cancer.
Reference
Finn RS, Gelmon KA, Ettl J, et al. Impact of prior treatment on palbociclib plus letrozole efficacy and safety in patients with estrogen receptor-positive/human epidermal growth factor receptor 2-negative (ER+/HER2-) first-line advanced breast cancer: a PALOMA-2 subgroup analysis. Presented at the 35th Annual Miami Breast Cancer Conference® (MBCC); March 8-11, Miami Beach, FL. Abstract 591.
Nurse Practitioners Weigh in on Data From the San Antonio Breast Cancer Symposium
January 16th 2023Loyda Braithwaite, MSN, RN, AGPCNP-BC, AOCNP; and Jamie Carroll, APRN, CNP, MSN, highlight presentations from the 2022 San Antonio Breast Cancer Symposium that will influence oncology nursing practice.



