Copanlisib Plus Rituximab May Be ‘Viable Option’ Across Indolent Non-Hodgkin Lymphoma
More data are needed regarding the duration of response associated with this combination to make it a “mainstream” treatment option for patients, according to an expert from the University of Michigan Rogel Cancer Center.
Copanlisib Plus Rituximab May Be ‘Viable Option’ Across Indolent Non-Hodgkin Lymphoma
Copanlisib (Aligopa) plus rituximab (Rituxan) proved to be safe and effective in the treatment of patients with relapsed indolent non-Hodgkin lymphoma (iNHL), compared with rituximab plus placebo, according to an expert from the University of Michigan Rogel Cancer Center.
Tycel J. Phillips, MD,, a clinical associate professor at the University of Michigan Rogel Cancer Center in Ann Arbor, discussed data from the ongoing CHRONOS-3 study (NCT02367040), which examined the combination of copanlisib and rituximab vs placebo plus rituximab in patients with relapsed iNHL, as part of an OncLive® Rapid Readout program. Results from the trial were originally presented at the American Association for Cancer Research Annual Meeting 2021.
The median progression-free survival (PFS) for patients with iNHL treated with copanlisib plus rituximab (n = 307) was 21.5 months (95% CI, 17.8-33.0) compared with 13.8 months (95% CI, 10.2-17.5) for the 151 patients in the rituximab plus placebo cohort (HR, 0.52; 95% CI, 0.39-0.68; P < .0001). The median duration of treatment was 8.3 months (range, 0.2-54.0) and 10.8 months (range, 0.2-46.6) in the copanlisib and rituximab and the rituximab plus placebo groups, respectively.
“Given that this study was mainly done in patients in a second-line setting, it does appear in this situation that copanlisib plus rituximab is a viable option for this patient population,” Phillips explained. “Ideally, the key to if this moves into the mainstream will be the duration of response of this treatment and, [specifically,] how durable some of the complete remissions are in this patient population. As of now, with the data we have, that information has not been extrapolated.”
Copanlisib, a selective, potent, intravenous (IV) pan-class I PI3K inhibitor, is approved as monotherapy for the treatment of patients with relapsed follicular lymphoma (FL) who have received at least 2 systemic therapies.
Copanlisib has predominant on-target activity against the PI3K-α and PI3K-σ isoforms. Rituximab monotherapy is recognized as a standard of care for patients with relapsed iNHL and a long remission after prior rituximab-based therapy, or for those who are unwilling to have or unfit for treatment with chemotherapy.
CHRONOS-3 is a randomized, doubleblinded, placebo-controlled, phase 3 study enrolling a total of 458 adult patients with indolent B-cell lymphoma. Patients included were those with relapsed disease following a previous rituximab, rituximab biosimilar, or anti-CD20 monoclonal antibody–containing regimen. Study participants also had to be progression and treatment free for at least 12 months since the previous rituximab-containing regimen or at least 6 months for patients who were unwilling or unfit to receive chemotherapy.
Patients were randomized 2:1 in favor of the experimental arm. In the copanlisib plus rituximab arm, patients received IV copanlisib at a dose of 60 mg on days 1, 8, and 15 of a 28-day cycle. Rituximab was given at a dose of 375 mg/m2 on days 1, 8, 15, and 22 during cycle 1, and on day 1 of cycles 3, 5, 7 and 9.
The dose scheduled was mirrored in the control arm, with placebo taking the place of copanlisib. Treatment in both arms contin-ued until disease progression or intolerance. Additionally, the investigators conducted a safety follow-up, active follow-up, and survival follow-up in both cohorts.
The primary end point of the study was PFS by central review. Secondary end points included objective response rate (ORR), duration of response (DOR), and safety, and tertiary end points include pharmacokinetics and biomarkers.
In the experimental group, 59.9% of patients presented with FL, 21.5% with marginal zone lymphoma (MZL), 11.4% with small lymphocytic lymphoma (SLL), and 7.2% with lymphoplasmacytic lymphoma (LPL) or Waldenström macroglobulinemia (WM) lymphoma. In the control group, 60.3% had FL, 19.2% had MZL, 9.9% were those with SLL, and 10.6% were those with LPL or WM.
Patients treated with copanlisib plus ritux-imab underwent dose reduction at a rate of 27% compared with 6.8% in the control group. Further, 75.2% and 56.8% of patients expe-rienced dose interruptions or delays in the experimental and control arms, respectively. Treatment discontinuation was reported for 31.9% of patients in the experimental arm vs 8.2% in the control arm because of adverse events.
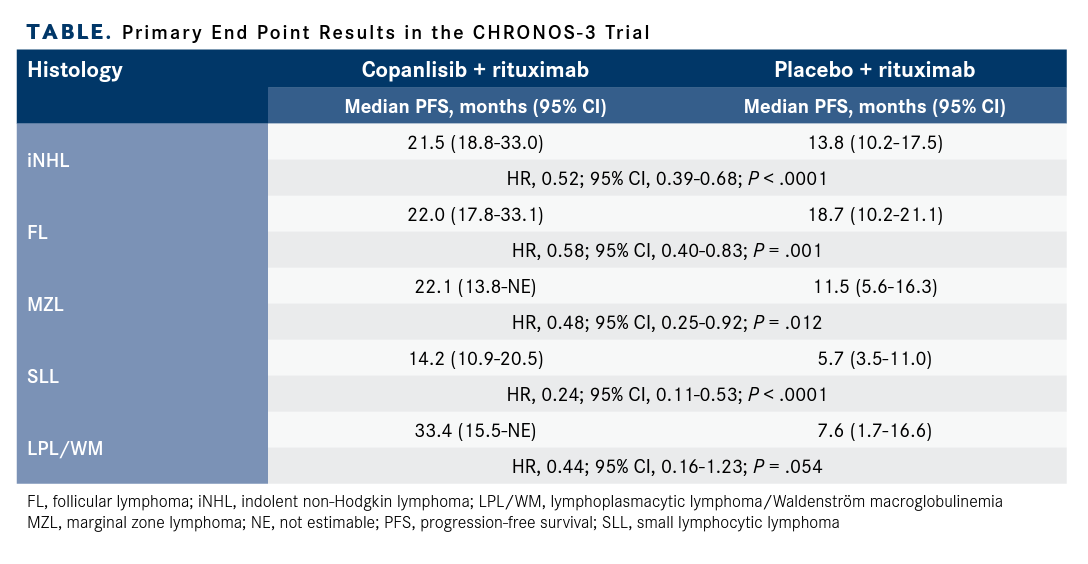
Subgroup data, stratified by histology, were also presented (TABLE). Patients with FL in the experimental arm had a median PFS of 22.2 months (95% CI, 17.8-33.1) vs 18.7 months (95% CI, 10.2-21.1) in the control arm (HR, 0.58; 95% CI, 0.40-0.83; P = .001). For patients with MZL, the median PFS was 22.1 (95% CI, 13.8-not estimable [NE]) and 11.5 months (95% CI, 5.6-16.3) in the experimental and control groups, respectively (HR, 0.48; 95% CI, 0.25-0.92; P = .012). Patients with SLL in the experimental arm had a PFS of 14.2 months (95% CI, 10.9-20.5), compared with 5.7 months (95% CI, 3.5-11.0) in the control arm (HR, 0.24; 95% CI, 0.11-0.53; P < .0001). The largest difference in PFS was in patients with LPL or WM: 33.4 months (95% CI, 15.5-NE) in the experimental arm vs 7.6 months (95% CI, 1.7-16.6) in the control arm (HR, 0.44; 95% CI, 0.16-1.23; P = .054).
Phillips, a clinical associate professor at the University of Michigan Rogel Cancer Center in Ann Arbor, discussed data from the ongoing CHRONOS-3 study (NCT02367040), which examined the combination of copanlisib and rituximab vs placebo plus rituximab in patients with relapsed iNHL, as part of an OncLive® Rapid Readout program. Results from the trial were originally presented at the American Association for Cancer Research Annual Meeting 2021.
The median progression-free survival (PFS) for patients with iNHL treated with copanlisib plus rituximab (n = 307) was 21.5 months (95% CI, 17.8-33.0) compared with 13.8 months (95% CI, 10.2-17.5) for the 151 patients in the rituximab plus placebo cohort (HR, 0.52; 95% CI, 0.39-0.68; P < .0001). The median duration of treatment was 8.3 months (range, 0.2-54.0) and 10.8 months (range, 0.2-46.6) in the copanlisib and rituximab and the rituximab plus placebo groups, respectively.
“Given that this study was mainly done in patients in a second-line setting, it does appear in this situation that copanlisib plus rituximab is a viable option for this patient population,” Phillips explained. “Ideally, the key to if this moves into the mainstream will be the duration of response of this treatment and, [specifically,] how durable some of the complete remissions are in this patient population. As of now, with the data we have, that information has not been extrapolated.”
Copanlisib, a selective, potent, intravenous (IV) pan-class I PI3K inhibitor, is approved as monotherapy for the treatment of patients with relapsed follicular lymphoma (FL) who have received at least 2 systemic therapies.
Copanlisib has predominant on-target activity against the PI3K-α and PI3K-σ isoforms. Rituximab monotherapy is recognized as a standard of care for patients with relapsed iNHL and a long remission after prior rituximab-based therapy, or for those who are unwilling to have or unfit for treatment with chemotherapy.
CHRONOS-3 is a randomized, doubleblinded, placebo-controlled, phase 3 study enrolling a total of 458 adult patients with indolent B-cell lymphoma. Patients included were those with relapsed disease following a previous rituximab, rituximab biosimilar, or anti-CD20 monoclonal antibody–containing regimen. Study participants also had to be progression and treatment free for at least 12 months since the previous rituximab-containing regimen or at least 6 months for patients who were unwilling or unfit to receive chemotherapy.
Patients were randomized 2:1 in favor of the experimental arm. In the copanlisib plus rituximab arm, patients received IV copanlisib at a dose of 60 mg on days 1, 8, and 15 of a 28-day cycle. Rituximab was given at a dose of 375 mg/m2 on days 1, 8, 15, and 22 during cycle 1, and on day 1 of cycles 3, 5, 7 and 9.
The dose scheduled was mirrored in the control arm, with placebo taking the place of copanlisib. Treatment in both arms contin-ued until disease progression or intolerance. Additionally, the investigators conducted a safety follow-up, active follow-up, and survival follow-up in both cohorts.
The primary end point of the study was PFS by central review. Secondary end points included objective response rate (ORR), duration of response (DOR), and safety, and tertiary end points include pharmacokinetics and biomarkers.
In the experimental group, 59.9% of patients presented with FL, 21.5% with marginal zone lymphoma (MZL), 11.4% with small lymphocytic lymphoma (SLL), and 7.2% with lymphoplasmacytic lymphoma (LPL) or Waldenström macroglobulinemia (WM) lymphoma. In the control group, 60.3% had FL, 19.2% had MZL, 9.9% were those with SLL, and 10.6% were those with LPL or WM.
Patients treated with copanlisib plus ritux-imab underwent dose reduction at a rate of 27% compared with 6.8% in the control group. Further, 75.2% and 56.8% of patients expe-rienced dose interruptions or delays in the experimental and control arms, respectively. Treatment discontinuation was reported for 31.9% of patients in the experimental arm vs 8.2% in the control arm because of adverse events.
Subgroup data, stratified by histology, were also presented (TABLE). Patients with FL in the experimental arm had a median PFS of 22.2 months (95% CI, 17.8-33.1) vs 18.7 months (95% CI, 10.2-21.1) in the control arm (HR, 0.58; 95% CI, 0.40-0.83; P = .001). For patients with MZL, the median PFS was 22.1 (95% CI, 13.8-not estimable [NE]) and 11.5 months (95% CI, 5.6-16.3) in the experimental and control groups, respectively (HR, 0.48; 95% CI, 0.25-0.92; P = .012). Patients with SLL in the experimental arm had a PFS of 14.2 months (95% CI, 10.9-20.5), compared with 5.7 months (95% CI, 3.5-11.0) in the control arm (HR, 0.24; 95% CI, 0.11-0.53; P < .0001). The largest difference in PFS was in patients with LPL or WM: 33.4 months (95% CI, 15.5-NE) in the experimental arm vs 7.6 months (95% CI, 1.7-16.6) in the control arm (HR, 0.44; 95% CI, 0.16-1.23; P = .054).
TABLE. Primary End Point Results in the CHRONOS-3 Trial
TABLE. Primary End Point Results in the CHRONOS-3 Trial
Overall, copanlisib plus rituximab elicited an ORR of 81%, with 34% of responders expe-riencing a complete response (CR). Patients treated with placebo and rituximab had an ORR of 48% and a 15% CR rate. At a median follow-up of 20.0 months, the median DOR was 20.4 months (range, 17.0-30.8) and 17.3 months (range, 11.8-25.3) in the experimental and control groups, respectively.
“A key point to this study, when looking at the benefits, is the safety profile,” Phillips said. “All patients in the [experimental arm of the] study had some treatment-emergent adverse event [TEAE].”
Common TEAEs included hyperglycemia, hypertension, and diarrhea. Hyperglycemia of any grade occurred in 69.4% of patients in the experimental group, with grade 3 occur-ring at a rate of 48.2% and grade 4 at a rate of 8.1%. In the control arm, hyperglycemia was experienced at any grade by 23.3% of patients and at grade 3 in 8.2%, with no patients having grade 4. Hypertension of any grade was seen in 49.2% of the experimental group vs 19.2% in the control group. Diarrhea of any grade occurred at a rate of 33.6% in the experimental cohort, compared with 9.6% in the control arm.
Pneumonitis was an AE of interest in the study. A total of 6.8% of patients in the experimental arm experienced pneumonitis of any grade; 2.0% of these were grade 3 events and 0.7% were grade 4. Patients in the control arm had pneumonitis of any grade at a rate of 1.4%, with 0.7% of these being grade 3 and no patients having grade 4 events.
In terms of TEAEs of any grade that led to discontinuation, 31.3% occurred in the experimental arm and 8.2% were seen in the control arm. Common TEAEs of any grade leading to discontinuation on the exper-imental side were pneumonitis (6.2%), hyperglycemia (2.6%), interstitial lung disease (1.3%), and pneumocystis jirovecii pneumonia (1.3%).
“Overall, this is a very good start,” Phillips said. “We are opening the window for this sort of treatment. As was demonstrated in this study, it does appear that the addition of rituximab did not add any new safety informa-tion or concerns when given with copanlisib. This was something we had not seen with a majority of the delta inhibitors, which have specifically all been given as single agents.”
References
- Matasar MJ, Capra M, Özcan M, et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021; 22(5):678-689. doi:10.1016/S1470-2045(21)00145-5
Shared Model of Care Post-HCT Offers Safe Follow-Up, Reduces Patient Burden
Published: March 19th 2025 | Updated: March 19th 2025Alternating post-HCT care between specialized facilities and local cancer centers produced noninferior non-relapse mortality and similar quality of life to usual care.


