NCCN's Just Bag It Campaign Seeks to Eliminate Fatal Vincristine Errors
In order to prevent near fatal intrathecal administering of vincristine, the NCCN has launched a campaign "Just Bag It." This campaign pushes for vincristine to be stored in IV drip bags to ensure vincristine is only administered intravenously.
NCCN’s Just Bag It Campaign Seeks to Eliminate Fatal Medical Error
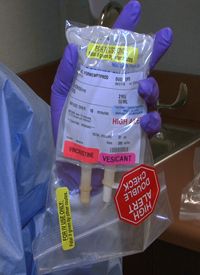
The National Comprehensive Cancer Network (NCCN) has launched a new “Just Bag It” campaign for the safe handling of vincristine (and other vinca alkaloids) in an effort to prevent a rare but fatal medical mistake when vincristine is administered intrathecally instead of intravenously.
Vincristine has been widely used since the 1960s, typically in conjunction with other chemotherapies. When it enters the blood, it is highly effective at blocking the growth of cancer cells by preventing their separation. However, vincristine is a neurotoxin that when given intrathecally causes profound neurotoxicity, leading to encephalopathy, ascending paralysis, and collapse. This is nearly always fatal, and in other instances leads to severe disability including paraparesis or tetraparesis.
Inadvertent intrathecal administration of vincristine has occurred in 125 reported cases in the United States and internationally, with the most recent documented case occurring over a year ago in Argentina, the NCCN reported in a press conference November 10. The organization noted that it is likely that more cases do exist, but were not reported, and so the true statistic for fatalities due to intrathecal administration of vincristine is unknown. There are currently no mandatory reporting laws, and physicians may not even recognize when the mistake occurs.
The Need for “Just Bag It”
The campaign calls for the adoption of a simple strategy for handling vincristine: always dilute vincristine in a 50 mL mini-IV drip bag to administer it intravenously and never put the vincristine into a syringe. With this approach, as Robert W. Carlson, MD, Chief Executive Officer of the NCCN, points out, “It makes it essentially impossible to actually give the vincristine intrathecally, because you need pressure in order to push the agents into the spinal fluid, and you don’t get adequate amounts of pressure with an IV.” Diluting vincristine into a mini IV-drip bag may entail a change in practice for some providers, but it is well worth the outcome of avoiding preventable deaths.
The NCCN has attempted to prevent this mistake in the past, with little effect. This mistake, and ways to prevent it, have been reported in medical literature for decades. The NCCN was involved in a 2001 study showing that diluting vincristine in an IV minibag was safer and released guidelines for labeling vincristine syringes with an explicit warning against intrathecal administration.
Drug labels for vincristine, such as Oncovin, Vincasar, and Marqibo, clearly state that vincristine should be administered intravenously only, never intrathecally. F. Marc Stewart, MD, acting division head of medical oncology at the University of Washington, adds that other resources have been available, warning against this very mistake:
“In 2008, the Best Practices Committee led the charge for NCCN to begin publishing chemotherapy order templates, which detail the most common regimens of many cancers and highlight safety parameters and special instructions. For example, the policy to dilute and administer vincristine in a minibag is called out in the safety parameters section.”
How Do Vincristine Mistakes Happen?
While it is uncommon, intrathecal administration of vincristine can occur for a number of reasons. In some cases, those administering the vincristine through a syringe simply do not read the label. As Michael R. Cohen, RPh, MS, ScD, DPS, FASHP, comments, “Not everybody does what they’re supposed to do, unfortunately, and it’s not like there’s necessarily an oversight agency to make sure that’s done.”
The NCCN notes that some clinicians administering the vincristine may not be familiar with the agent. When they administer other chemotherapeutic agents intrathecally as is appropriate, they will assume the vincristine—which is already in a syringe—is supposed to be administered intrathecally as well.
Additionally, performing a lumbar puncture to administer intrathecal chemotherapy takes a fair amount of focus and attention on the sterile procedure. If the vincristine syringe is within reach, there is no way to feel the difference between the vincristine and the other chemotherapeutic agents, and so it can be picked up and administered intrathecally. When the vincristine is in an IV bag, this particular error is eliminated.
Emergency Treatment for Intrathecal Vincristine
There is only 1 emergency treatment for accidental intrathecal administration of vincristine, but it is not very effective. This antidote involves direct aspiration of the fluid in the spinal intrathecal space to reduce the binding of vincristine to the neurotubules. In most cases, patients die despite this treatment, and those who survive following supportive care therapy suffer severe and irreversible central nervous system toxicity and motor dysfunction.
The Vincristine Safe Handling Campaign’s Progress
So far, adoption of this campaign is going well, Carlson reported. “All 27 of the NCCN member institutions have adopted policies that are consistent with our vincristine guidelines requiring that the vincristine be drawn up or prepared only in an IV minibag. This policy is also consistent with that of the Institute of Safe Medication Practices, the Joint Commission that accredits hospitals, the World Health Organization, and the Oncology Nursing Society.”
However, there is some resistance. While the NCCN member institutions were quick to pick up these guidelines, other institutions are less so. Bagging vincristine costs about $1 to $2, but this is a small cost by any definition when stacked up against the lives that are potentially on the line. “I think that cost is probably not an issue here,” says Stewart, “I think probably what is an issue is just the age-old habit of putting vincristine in a syringe.”
There is also a small risk of extravasation, or the leaking of a chemotherapy drug into the tissue surrounding the intravenous administration site. But research shows that the risk of extravasation is extremely low (<.05%) regardless of how vincristine is administered. When resident nurses administer vincristine, they tend to prefer a syringe intravenous injection so that they can monitor for extravasation, but it may actually be easier to monitor patients when they are using an IV bag.
Inadvertent administration of vincristine intrathecally is not just a problem in US hospitals. “This is an international problem as well. We really hope that by NCCN working on this project that it will not only get Canadian and North American attention but also attention internationally,” said Cohen.
Click here for more on the NCCN’s Just Bag It! Campaign for Safe Vincristine Handling.



